Lonquex - Lipegfilgrastim 10mg/ml - 1 Seringa Segurança Preenchida 0,6ml SC (2 A 8C) - Teva - ÁgilMed - Medicamentos Especiais e Nutrição Clínica
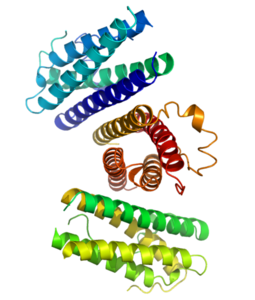
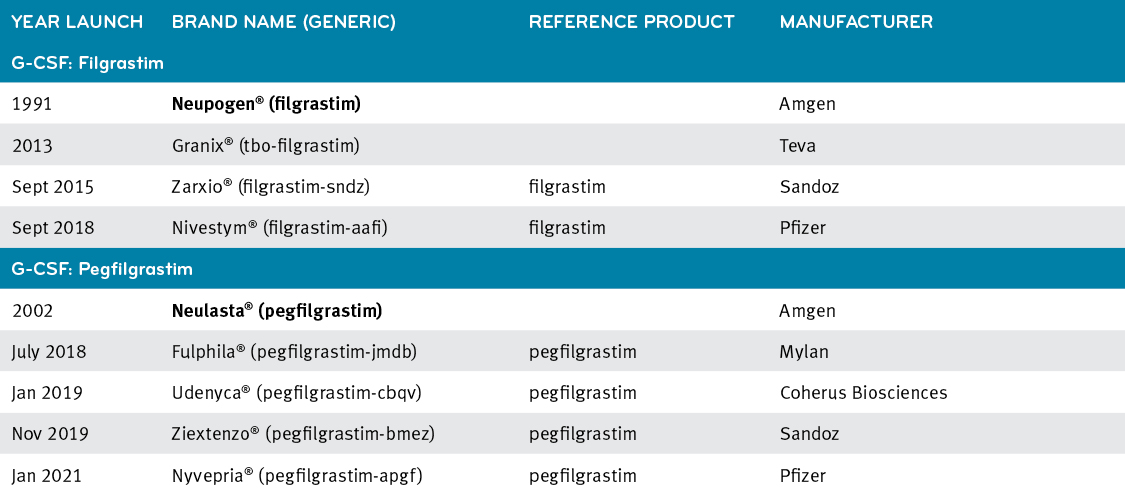
Key concepts and critical issues on epoetin and filgrastim biosimilars. A position paper from the Italian Society of Hematology, Italian Society of Experimental Hematology, and Italian Group for Bone Marrow Transplantation

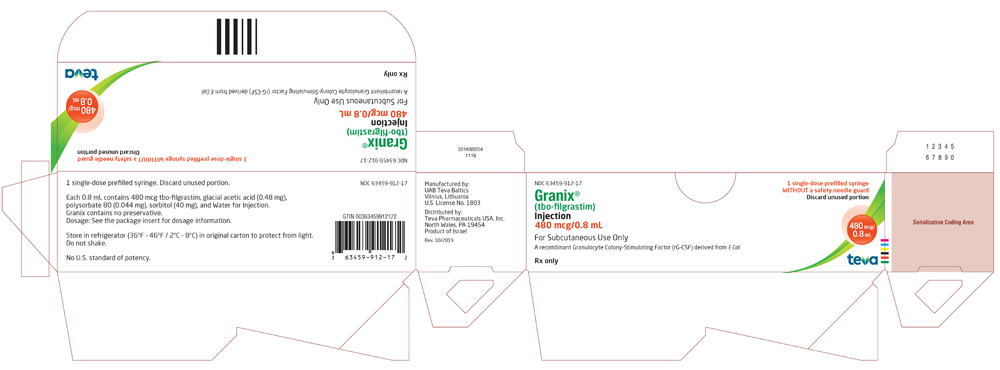
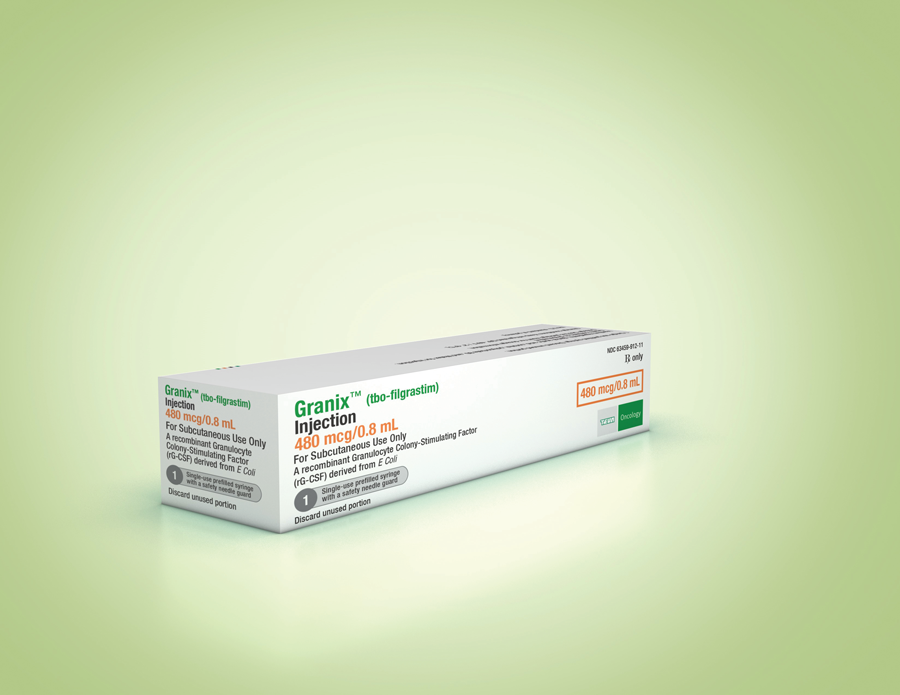
Granix® Hematopoietic Agent TBO-Filgrastim, Preservative Free 480 mcg / 0.8 mL Subcutaneous Injection Prefilled Syringe 5 Syringes - Suprememed